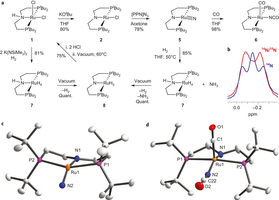
An iridium(III/IV/V) redox series featuring a terminal imido complex with triplet ground state
The iridium(III/IV/V) imido redox series [Ir(NtBu){N(CHCHPtBu2)2}]0/+/2+ was synthesized and examined spectroscopically, magnetically, crystallographically and computationally. The monocationic iridium(IV) imide exhibits an electronic doublet ground state with considerable ‘imidyl’ character as a result of covalent Ir–NtBu bonding. Reduction gives the neutral imide [Ir(NtBu){N(CHCHPtBu2)2}] as the first example of an iridium complex with a triplet ground state. Its reactivity with respect to nitrene transfer to selected electrophiles (CO2) and nucleophiles (PMe3), respectively, is reported.
Figure: Computed state-energy diagram of [Ir(NtBu)(PNP)] . Relative energies of the lowest non-relativistic (left) and spin–orbit states (right) in red (cm−1) and corresponding |S, MS〉 labels in blue.
Chem. Sci. 9, 4325–4332 (2018)
in cooperation with Prof. Schneider (Göttingen), Prof. de Bruin (Amsterdam) and Prof. van Slageren (Stuttgart)
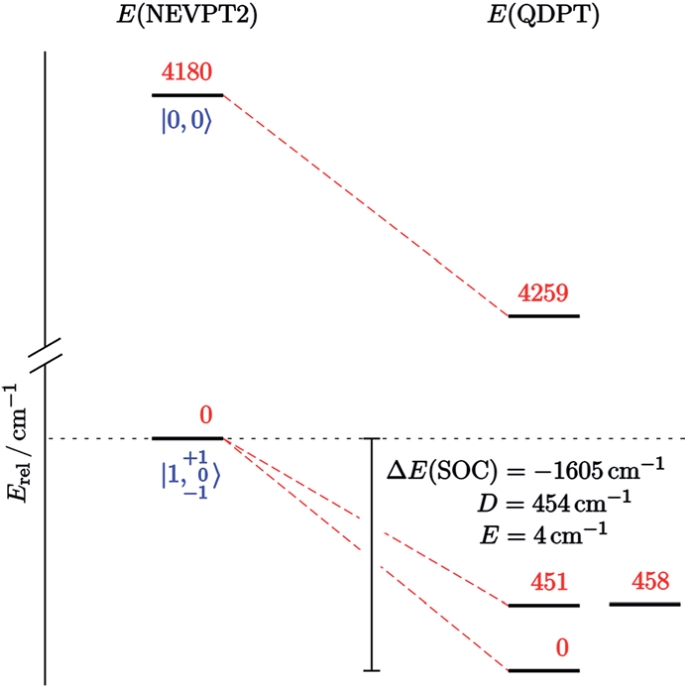
A square-planar osmium(II) complex
Reduction of the pincer complex [OsIIICl2(PNP)] (PNP = N(CHCHPtBu2)2) affords the isolation and full characterization of an osmium(II) complex with square-planar coordination geometry, i.e. [OsIICl(PNP)]. Spectroscopic, structural and magnetic data in combination with multireference computations indicate strong temperature independent paramagnetism, which arises from an energetically well separated ground state that mixes with excited states through spin–orbit coupling.
Figure: State-energy diagram for [Os[OsIICl(PNP)] based on NEVPT2/SA-CASSCF(16,10) computations. Non-relativistic energies of the lowest four states are shown with their corresponding |S,MS〉 label (left). The lowest nine spin–orbit states (right) are complemented with selected contributions (weights >10%) of the corresponding spin-free states (dashed red lines).
Chem. Commun. 53, 5511–5514 (2017)
in cooperation with Prof. Schneider (Göttingen)
Dinitrogen Splitting Coupled to Protonation
Acid splits: Protonation of an N2‐bridged dimolybdenum complex in the pincer periphery results in splitting into MoV nitrides. This proton‐coupled metal‐to‐ligand charge transfer reaction provides a mechanism to control the thermochemistry and kinetics of N−N bond cleavage with proton‐responsive ligands.
Angew. Chem. Int. Ed. 56, 5872–5876 (2017)
in cooperation with Prof. Schneider (Göttingen)
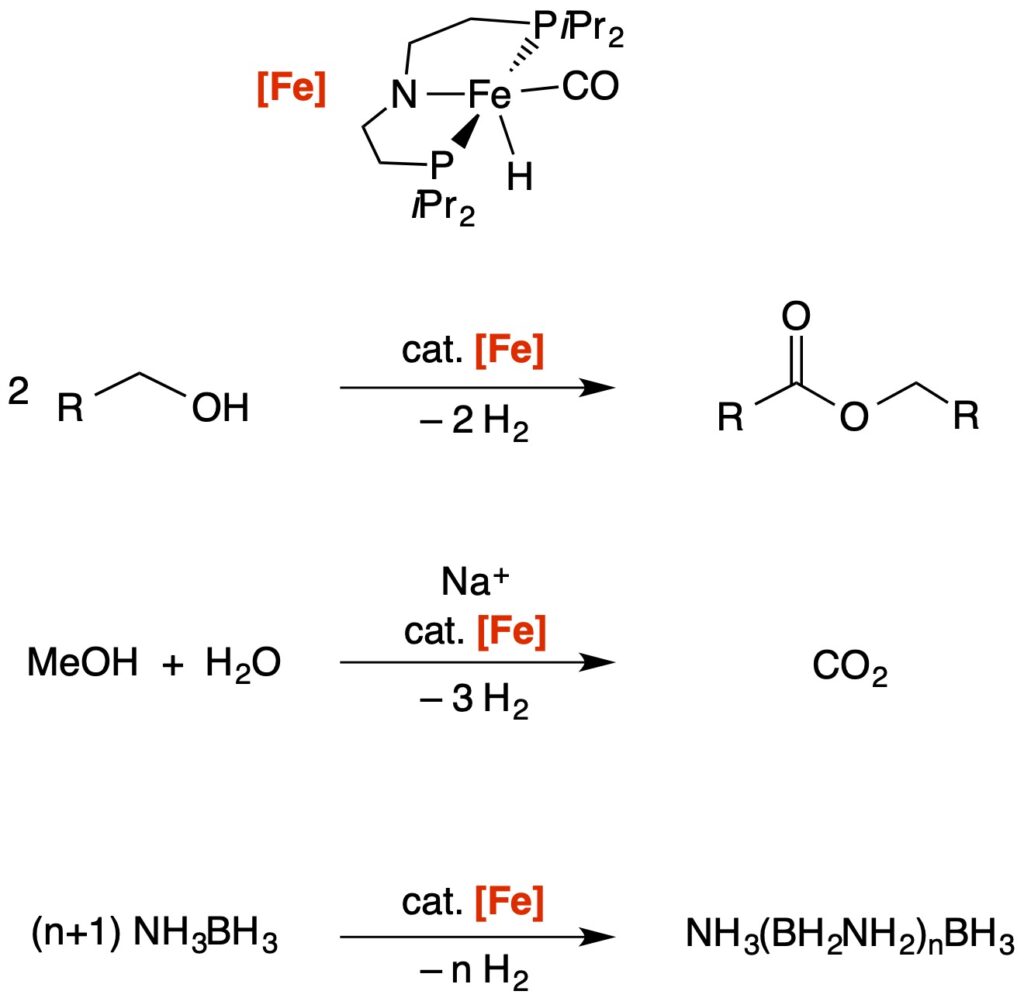
Fe-PNP Mediated Dehydrogenation Catalysis
Chemical hydrogen storage materials: The pincer-supported iron catalyst [Fe] was successfully employed in highly-efficient dehydrogenation reactions of different substrates. The development of iron-based homogeneous catalysts for these applications (i.e. dehydrogenation of potential chemical hydrogen storage materials) is a major advancement in this field, since conventional approaches used precious or heavy metals exclusively.
ACS Catal. 5, 7214 –7217 (2015)
ACS Catal. 5, 2404 –2415 (2015); Highlighted in ACS Catal. 5, 5584 –5585 (2015)
ACS Catal. 4, 3994 –4003 (2014)
in cooperation with Prof. Schneider (Göttingen), Prof. Schmedt a. d. Günne (Siegen), Prof. Bernskoetter (Brown University), Prof. Hazari (Yale University), and Prof. Jones (Rochester)
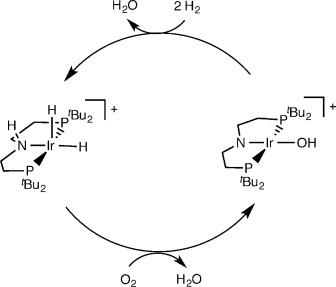
Oxygen Reduction with a Bifunctional Iridium Dihydride Complex
A mononuclear mechanism: The oxygen-reduction reaction (ORR) with an iridium dihydride results in formation of an unusual square-planar iridium(III) hydroxide and water. The dihydride is regenerated with H2 in a quasi-catalytic synthetic cycle. Experimental and computational studies are in agreement with a four-electron ORR mechanism at a single metal site.
Angew. Chem. Int. Ed. 54, 15271–15275 (2015)
Angew. Chem. 127, 15486–15490 (2015)
in cooperation with Prof. Schneider (Göttingen)
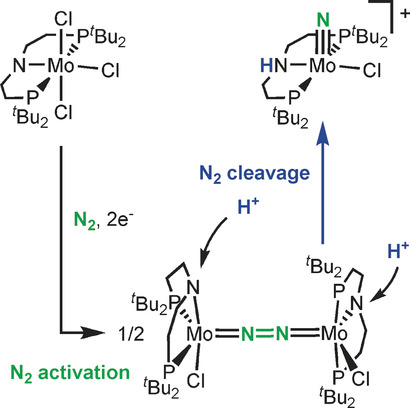
Ammonia formation by metal–ligand cooperative hydrogenolysis of a nitrido ligand
Bioinspired hydrogenation of N2 to ammonia at ambient conditions by stepwise nitrogen protonation/reduction with metal complexes in solution has experienced remarkable progress. In contrast, the highly desirable direct hydrogenation with H2 remains difficult. In analogy to the heterogeneously catalysed Haber–Bosch process, such a reaction is conceivable via metal-centred N2 splitting and unprecedented hydrogenolysis of the nitrido ligands to ammonia. We report the synthesis of a ruthenium(IV) nitrido complex. The high nucleophilicity of the nitrido ligand is demonstrated by unusual N–C coupling with π-acidic CO. Furthermore, the terminal nitrido ligand undergoes facile hydrogenolysis with H2 at ambient conditions to produce ammonia in high yield. Kinetic and quantum chemical examinations of this reaction suggest cooperative behaviour of a phosphorus–nitrogen–phosphorus pincer ligand in rate-determining heterolytic hydrogen splitting.
Nature Chem. 3, 532–537 (2011)
Highlighted in Nature Chem. 3, 502–504 (2011), Chemistry World, May 25, 2011
in cooperation with Prof. Schneider (Göttingen), and Dr. Herdtweck (München)